Sponsor Ads
Non-China Vape, 510 Cartridges & Battery Device Maker
If you are going for a vape manufacturer out of China we are you best choice. We offer a alternative vape production location with a very competitive price. You can save money by buying directly from us the manufacturer, without any middlemen or extra fees. You can also enjoy discounts for bulk orders and special offers for long term & loyal customers.
We offer small trial orders where you can test the quality and performance of the products before placing a large order. Fast shipping and cheaper shipping cost from Malaysia and Singapore ports. You don't have to wait long to receive your products.
Contact Us!
Contribute for our website Maintenance! We want to keep it free for all visitors.
Trending Best Sellers
Safety of COVID-19 vaccines – what we need to know
Safety of COVID-19 vaccines – what we need to know
How do we know that COVID-19 vaccines are safe?
There are strict protections in place to help ensure the safety of all COVID-19 vaccines. Before receiving validation from WHO and national regulatory agencies, COVID-19 vaccines must undergo rigorous testing in clinical trials to prove that they meet internationally agreed benchmarks for safety and effectiveness.
Unprecedented scientific collaborations have allowed COVID-19 vaccine research, development, and authorizations to be completed in record time – to meet the urgent need for COVID-19 vaccines while maintaining high safety standards. As with all vaccines, WHO and regulatory authorities will continuously monitor the use of COVID-19 vaccines to confirm that they remain safe for all who receive them.
What are the side effects of COVID-19 vaccines?
Like any vaccine, COVID-19 vaccines can cause mild side effects, such as a low-grade fever or pain or redness at the injection site. Most reactions to vaccines are mild and go away within a few days on their own. More serious or long-lasting side effects to vaccines are possible but extremely rare. Vaccines are continually monitored to detect rare adverse events.
Reported side effects to COVID-19 vaccines have mostly been mild to moderate and short-lasting. They include: fever, fatigue, headache, muscle pain, chills, diarrhoea, and pain at the injection site. The chances of any of these side effects following vaccination differ according to the specific COVID-19 vaccine.
What is the link between COVID-19 vaccines and allergic reactions?
WHO is aware of reports of severe allergic reactions in a small number of people who received a COVID-19 vaccine. A severe allergic reaction – such as anaphylaxis – is a potential but rare side effect with any vaccine. In persons with a known risk, such as previous experience of an allergic reaction to a previous dose of the vaccine or any of the known components in the vaccine, precautions may need to be taken.
WHO recommends that healthcare providers assess patient medical history to determine if a patient is at risk for severe allergic reaction to a COVID-19 vaccine. All immunization providers should be trained to recognize severe allergic reactions and take practical steps to treat such reactions if they occur.
COVID-19 vaccine use will be closely monitored by national authorities and international bodies, including WHO, to detect serious side effects, including any unexpected side effects. This will help us better understand and manage the specific risks of allergic reactions or other serious side effects to COVID-19 vaccines that may not have been detected during clinical trials, ensuring safe vaccination for all.
What happens if an adverse event is reported?
As with any vaccine, it is essential to closely monitor the safety and efficacy of COVID-19 vaccines as they are delivered. If a problem is reported following vaccination, a thorough investigation should take place.
During these investigations, it is extremely rare that health problems are found to be caused by the vaccine itself. Adverse events are most often found to be coincidental and may be entirely unrelated to vaccination. Sometimes they are related to how the vaccine has been stored, transported, or administered. Such errors can be prevented by better training health workers and strengthening supply chains.
In the very rare cases where a genuine adverse reaction is suspected, the vaccine may be suspended from use. Further investigations will take place to determine what exactly caused the event, and corrective measures will be put in place. WHO works with vaccine manufacturers, health officials and other partners to monitor any safety concerns and potential side effects on an ongoing basis.
Is Corona Vaccine Safe?
Guidance says there are no safety concerns about giving jabs to people with "long" Covid either. But people who are currently unwell with Covid-19 should not receive the vaccine until they have recovered.
Trending Best Sellers
Is Corona Vaccine Safe? What do we know about its safety?
The U.S. vaccine safety system works to make sure that all vaccines are as safe as possible. Safety has been a top priority as federal agencies work with vaccine manufacturers to develop and authorize a COVID-19 vaccine. Here are some key areas that are part of a COVID-19 vaccine development, review and authorization:
- Careful testing. All vaccines go through clinical trials to test safety and effectiveness. For the COVID-19 vaccine, the Food and Drug Administration (FDA) set up rigorous standards for vaccine developers to meet. This infographic from the National Institutes of Health shows the four phases a vaccine must go through before it is released to the public.
- Authorization for emergency use. Vaccines that meet FDA safety and effectiveness standards can be made available in the United States by approval or by emergency use authorization (EUA). An EUA provides temporary authorization of a vaccine or medication under emergency situations, such as the coronavirus pandemic.
- Continuous monitoring for problems and side effects. Once a vaccine is authorized for use, monitoring continues, with systems in place to track problems or side effects that were not detected during the clinical trials. For the COVID-19 vaccine, the FDA and the Centers for Disease Control and Prevention (CDC) are expanding their vaccine monitoring. If there are problems with the vaccine, they are most likely to emerge early in the testing process when they can be identified and addressed.
You can learn more from the CDC about the safety steps in place for the COVID-19 vaccine.
Is Corona Vaccine Safe? What CDC has to say!
Safety of COVID-19 Vaccines
The U.S. Food and Drug Administration (FDA) has granted Emergency Use Authorizations (EUA) for two COVID-19 vaccines which have been shown to be safe and effective as determined by data from the manufacturers and findings from large clinical trials. These data demonstrate that the known and potential benefits of this vaccine outweigh the known and potential harms of becoming infected with the coronavirus disease 2019 (COVID 19).
Clinical Trials
Clinical trials are being conducted to evaluate additional COVID-19 vaccines in many thousands of study participants. These trials will generate scientific data and other information that will be used by FDA to determine vaccine safety and effectiveness. Clinical trials on all COVID-19 vaccine candidates are being conducted according to the rigorous standards set forth by FDA in their June 2020 guidance document, Development and Licensure of Vaccines to Prevent COVID-19external icon. If FDA determines that a vaccine meets its safety and effectiveness standards, it can make these vaccines available for use in the United States by approval or through an EUA.
After FDA determines that a COVID-19 vaccine candidate is safe and effective, the Advisory Committee on Immunization Practices (ACIP), a committee comprising medical and public health experts, reviews available data before making vaccine recommendations to CDC. Learn more about how CDC is making COVID-19 vaccine recommendations.
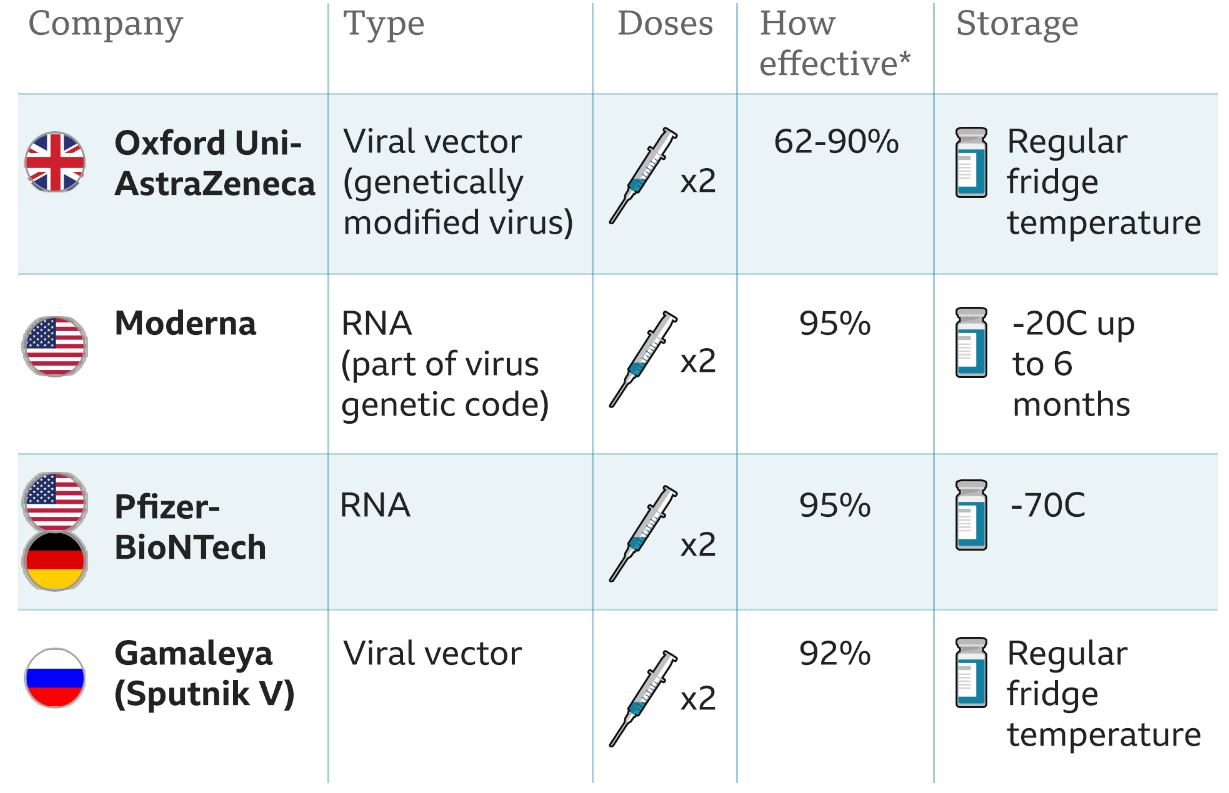
Credit: BBC Health
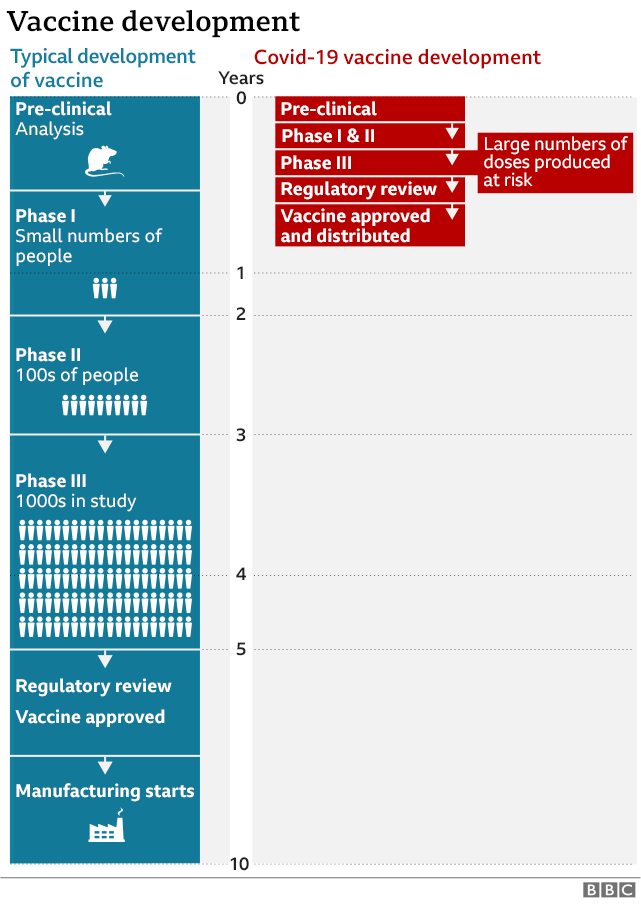
Credit: BBC Health
Disclaimer: the designations employed and the presentation of the material in publications listed in this database does not imply the expression of any opinion whatsoever on the part of NosePiercings.Com concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted and dashed lines on maps represent approximate border lines for which there may not yet be full agreement.
The mention of specific companies or of certain manufacturers’ products in publications listed in the database does not imply that they are endorsed or recommended by NosePiercings.Com in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters.
By listing publications in this database and providing links to external sites does not mean that NosePiercings.Com endorses or recommends those publications or sites, or has verified the content contained within them. The database has been compiled without warranty of any kind, either expressed or implied. The responsibility for the interpretation and use of publications included in this database lies with the reader. In no event shall NosePiercings.Com be liable for damages arising from its use.
Comments
What you think?
Recent Articles
-
Riche Niche: Health | Lifestyle | Fashion | Marketing | Technology
Mar 14, 25 09:18 AM
Our Riche Niche blog is the easiest way to stay up-to-date with the latest news, trends and articles published on this site. -
The Therapeutic Potential of Medical Cannabis Vaporization
Aug 05, 24 09:32 PM
The use of medical cannabis has been a subject of much debate and research over the years. With the growing acceptance of cannabis for medical purposes, various methods of administration have been exp… -
Amazon Spring Sale: A Season of Spectacular Savings
Mar 18, 24 08:38 AM
Amazon Spring Sale: A Season of Spectacular Savings -
Understanding Nose Piercing Types: A Guide for Teens
Mar 16, 24 09:19 AM
Explore the rising trend of nose piercings among teenagers, understanding the various types and their cultural implications for a stylish appeal. -
Infected Nose Piercing
Mar 16, 24 09:18 AM
You can expect symptoms of infected nose piercing to resemble any other kind of body piercing infection. -
EMS manufacturing services in Malaysia
Mar 09, 24 10:33 PM
Malaysia is one of the leading countries in Southeast Asia that offers EMS manufacturing services to both local and international clients. -
Laundry Business: The Need for Payment System Upgrades
Mar 08, 24 11:14 AM
Discover the benefits of upgrading your laundry business's payment system. Enhance efficiency, increase profits, and improve customer convenience. -
Nose Peircing Store
Feb 18, 24 02:38 AM
A collection of latest at our nose peircing store. -
How to Choose the Right Coffee Maker for Your Needs
Feb 18, 24 02:12 AM
We'll compare the pros and cons of four common types of coffee makers: drip, French press, espresso, and vacuum. We'll also give you some tips on how to choose the right one based on your preferences… -
Emulate Celebrities with Nose Piercings
Feb 06, 24 08:13 AM
Discover the celebrities with nose piercing and get inspired for your next piercing! From studs to septum rings, our list has it all. Read more! -
Types of Nose Rings
Feb 06, 24 08:11 AM
Types of Nose Rings -
Is my nose piercing ring is sinking in?
Feb 06, 24 08:10 AM
Is my nose piercing ring is sinking in? Or just swollen? -
Dry Herb Vape Pens-Discover the Advantages of Malaysian Made
Feb 04, 24 12:39 PM
Choose our non-China dry herb vape pen for its high production standards, strict quality control, and excellent craftsmanship. -
Trinity Nose Ring A Unique Fashion Statement
Feb 03, 24 08:36 PM
Explore the world of trinity nose rings, a unique piece of jewelry that adds elegance and style to your look. Understand the different types and choose the right one for you. -
Redefining Beauty: The Rise of Nose Piercing Trend in the USA
Feb 02, 24 08:34 AM
Explore the evolution of the nose piercing trend in the USA, from ancient tradition to modern expression of individuality.