Sponsor Ads
Non-China Vape, 510 Cartridges & Battery Device Maker
If you are going for a vape manufacturer out of China we are you best choice. We offer a alternative vape production location with a very competitive price. You can save money by buying directly from us the manufacturer, without any middlemen or extra fees. You can also enjoy discounts for bulk orders and special offers for long term & loyal customers.
We offer small trial orders where you can test the quality and performance of the products before placing a large order. Fast shipping and cheaper shipping cost from Malaysia and Singapore ports. You don't have to wait long to receive your products.
Contact Us!
Contribute for our website Maintenance! We want to keep it free for all visitors.
Trending Best Sellers
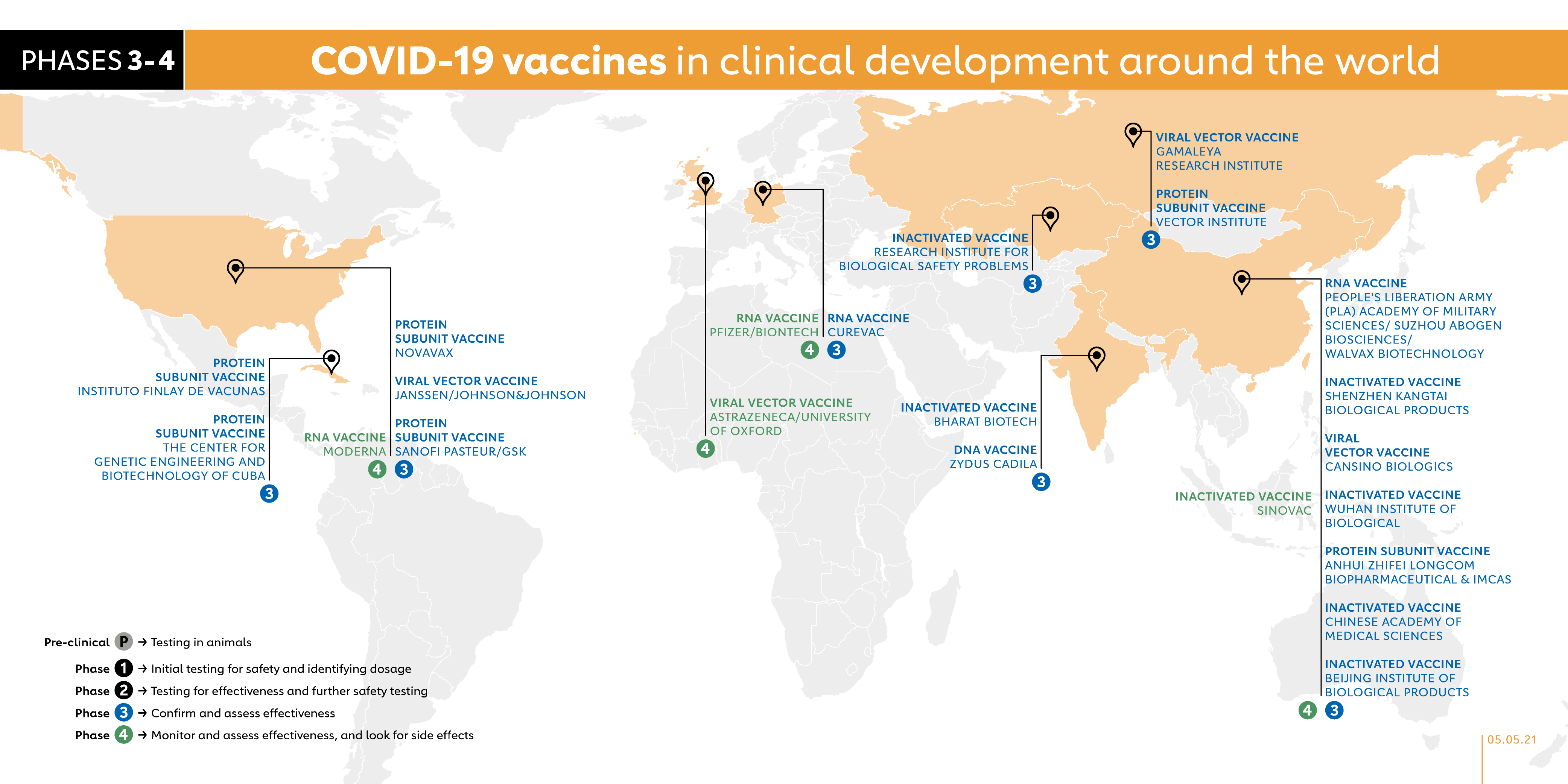
The COVID-19 vaccine race – weekly update
Trending Best SellersScientists around the world are working faster than ever to develop and produce vaccines that can stop the spread of COVID-19. Since the emergence of this novel coronavirus in December 2019, more than a dozen vaccines have started to be rolled out. Here is an at-a-glance overview of those vaccines and recent developments of those vaccine candidates in clinical trials.
5 May 2021
RECENT UPDATES
- US-based Moderna TX and the Scientific and Technological Research Council of Turkey each launched phase 1 trials for their vaccine candidates. This is Moderna’s third and Scientific and Technological Research Council’s second COVID-19 vaccine candidate currently in clinical trials.
- The National Vaccine and Serum Institute, China and Baltimore-based Elixirgen Therapeutics each launched phase 1/2 trials for their vaccine candidates.
- Kazakhstan began administering its homegrown QazVac vaccine to the general public, bringing the total number of vaccines being offered to a general population to 14 candidates.
- Around the world, there are now 96 COVID-19 vaccine candidates undergoing clinical trials and 184 candidates in pre-clinical development.
When candidate vaccines make it to human clinical trials, they first go through phase 1 trials primarily to test the vaccine’s safety, determine dosages and identify any potential side effects in a small number of people. Phase 2 trials further explore safety and start to investigate efficacy on larger groups. Phase 3 trials, which few vaccines ever make it to, are much larger, involving thousands or tens of thousands of people, to confirm and assess the effectiveness of the vaccine and test whether there are any rare side effects that only show up in large groups. The final stage, phase 4 trials, is conducted after national regulatory approval and involves further monitoring in a wide population over a longer timeframe as a form of post-marketing surveillance (pharmacovigilance). The World Health Organization (WHO) lists candidates at various stages of clinical trials.
Here is a slightly more in-depth look at the candidate vaccines that are in phase 1 trials or beyond.
- MODERNA (USA)
RNA VACCINE
The final trial results confirm this vaccine has a 94% efficacy, and the data has been sent to regulators around the world. As with the Pfizer vaccine, this RNA vaccine will need to be kept in ultra-cold freezers. The vaccine has been developed by Moderna, in Cambridge, Massachusetts, and funded by the National Institute of Allergy and Infectious Diseases (NIAID), which is part of the US National Institutes of Health. The vaccine was tested in phase 1 trials on volunteers at the Kaiser Permanente Washington Health Research Institute in Seattle. Moderna has run phase 2 trials on participants of a wide range of ages and started phase 3 trials in July 2020. The final trial enrolled 30,000 healthy people from across the United States. In February, a phase 4 trial was launched as part of a national cohort study in collaboration with the Danish Ministry of the Interior and Health.
- ASTRAZENECA/UNIVERSITY OF OXFORD (UK)
VIRAL VECTOR VACCINE
The ChAdOx1 vaccine, developed by the University of Oxford, has a vaccine efficacy of up to 90% and has been granted emergency use authorisation by the European Medicines Agency as well as national regulators in the UK, Argentina, India, Mexico, Brazil and Pakistan. Although the efficacy is slightly lower than the Moderna and Pfizer vaccines, it is fridge-stable meaning that it can be easily transported anywhere in the world. It was also found to be 100% effective against severe disease. At about US$ 4 per dose, it is also a fraction of the cost of others that are around US$ 26 per dose. It was tested in phase 3 clinical trials with more than 10,000 people from across the UK, including children and older people. The vaccine was also tested in Brazil, the United States and India and South Africa started the first COVID-19 vaccine trial in Africa. In February 2021, a phase 4 trial was launched as part of a national cohort study in collaboration with the Danish Ministry of the Interior and Health.
In March 2021, the University of Oxford registered a further phase 1 trial in the UK with 30 adult participants to investigate the delivery of its ChAdOx1 vaccine using a nasal spray. ChAdOx1 is currently being delivered by intramuscular injection as part of the UK’s national rollout. By using a different technique that administers the vaccine to the site of infection, researchers at Oxford intend to investigate whether this results in enhanced protection, especially against transmission and mild disease.
- PFIZER/BIONTECH (GERMANY)
RNA VACCINE
In December 2020, the UK became the first country in the world to approve this vaccine and began rolling out an initial 800,000 doses at the start of the month. BioNTech, working together with Pfizer, started testing its BNT162 vaccine in humans in global trials, initially in Germany, and then started trials in the USA. BioNTech has also entered into a € 100 million debt financing agreement with the European Investment Bank in order to scale-up the production of the vaccine in Europe. On 27 July 2020, it announced the launch of a phase 2/3 trial with 30,000 volunteers in the USA and other countries including Argentina, Brazil and Germany. In September, it said it would expand its phase 3 US trial to 43,000 participants. At the start of October 2020, BioNTech and Pfizer started recruiting for a phase 3 trial in South Africa and by early November had reported promising interim results. In its final efficacy analysis, its data showed a vaccine efficacy rate of 95% (even in adults over 65 years efficacy was more than 94%, which is reassuring as older people don’t always have a strong immune response to vaccines).
- SINOVAC (CHINA)
INACTIVATED VACCINE
Sinovac conducted phase 3 trials involving volunteers in Brazil, Indonesia and Turkey. Although it is not yet approved by regulators, shipments have already arrived in Indonesia, ready for rollout. A report in July said that the Chinese government has given the Sinovac vaccine emergency approval for limited use. The city of Jiaxing has reportedly offered the vaccine to health workers and other high-risk groups for US$ 60. The company began phase 4 trials in February 2021.
Source:
GAVI.ORG
https://www.gavi.org/vaccineswork/covid-19-vaccine-race
WHO
https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines
Watch for symptoms
People with COVID-19 have had a wide range of symptoms reported – ranging from mild symptoms to severe illness. Symptoms of coronavirus may appear 2-14 days after exposure to the virus. People with these symptoms may have COVID-19:
Fever or chills
Cough
Shortness of breath or difficulty breathing
Fatigue
Muscle or body aches
Headache
New loss of taste or smell
Sore throat
Congestion or runny nose
Nausea or vomiting
Diarrhea
This list does not include all possible symptoms of coronavirus. CDC will continue to update this list as we learn more about COVID-19.
Source: CDC
- Protect yourself: advice for the public
- Myth busters
- Questions and answers
- Situation reports
- All information on the COVID-19 outbreak
- Coronavirus Vaccine
- What is the Coronavirus Vaccine
- All About Covid-19
- Covid-19 Symptoms
Other resources on coronavirus disease (COVID-19)
- BMJ
- Cambridge University Press
- Centers for Disease Control and Prevention
- Chinese Medical Association
- Cochrane
- Elsevier
- European Centre for Disease Prevention and Control (ECDC)
- JAMA Network
- The Lancet
- LITCOVID: US National Library of Medicine
- New England Journal of Medicine
- Oxford University Press
- PLOS
- Public Health England
- Science
- Springer Nature
- SSRN (Preprints)
- Wiley
Important Covid-19 Links
This Coronavirus pages are intended solely to inform our clients and online visitors about coronavirus/COvid-19. All facts are from reliable sources like WHO, CDC and respective Government Health Agencies. Readers can either read through the facts which we summarized in this page or go direct to source through the link listed at the bottom of each page.
- Safety of COVID-19 vaccines-what we need to know!
- Coronavirus - Stay Informed
- Covid 19
- Covid 19 Symptoms
- Coronavirus Covid 19 Questions and Answers
- Coronavirus disease (COVID-19) advice for the public
- Coronavirus Vaccine - All you need to know!
- Covid 19 Testing Near Me
- Coronavirus Vaccine
- What is the Coronavirus Vaccine
- Coronavirus vaccine update - There are more than 100 vaccines!
- How long does coronavirus last - Stay Informed!
- All About Covid-19
- Covid-19 Symptoms
- Michigan Coronavirus _ Stay Informed and Stay Safe!
- Coronavirus California - Stay Informed & Stay Safe
- Florida coronavirus - Stay Informed!
- What is the coronavirus vaccine - Stay Informed
- Covid 19 Vaccine - Stay Informed with the latest Development!
- Protect yourself: advice for the public
- Myth busters
- Questions and answers
- Situation reports
- All information on the COVID-19 outbreak
References:
1. WHO Coronavirus disease (COVID-19) pandemic
2. CDC Centers for Disease and Prevention Control
3. Canada Coronavirus disease (COVID-19)
4. Health.com
5. Harvard Health Publishing Harvard Medical School
7. Wikipedia COVID-19 pandemic
Supply Chain
1) Nitrile Powder-Free Examination Gloves
3) Blue Nitrile Disposable Gloves Powder-Free
Comments
What you think?
Recent Articles
-
Riche Niche: Health | Lifestyle | Fashion | Marketing | Technology
Mar 14, 25 09:18 AM
Our Riche Niche blog is the easiest way to stay up-to-date with the latest news, trends and articles published on this site. -
The Therapeutic Potential of Medical Cannabis Vaporization
Aug 05, 24 09:32 PM
The use of medical cannabis has been a subject of much debate and research over the years. With the growing acceptance of cannabis for medical purposes, various methods of administration have been exp… -
Amazon Spring Sale: A Season of Spectacular Savings
Mar 18, 24 08:38 AM
Amazon Spring Sale: A Season of Spectacular Savings -
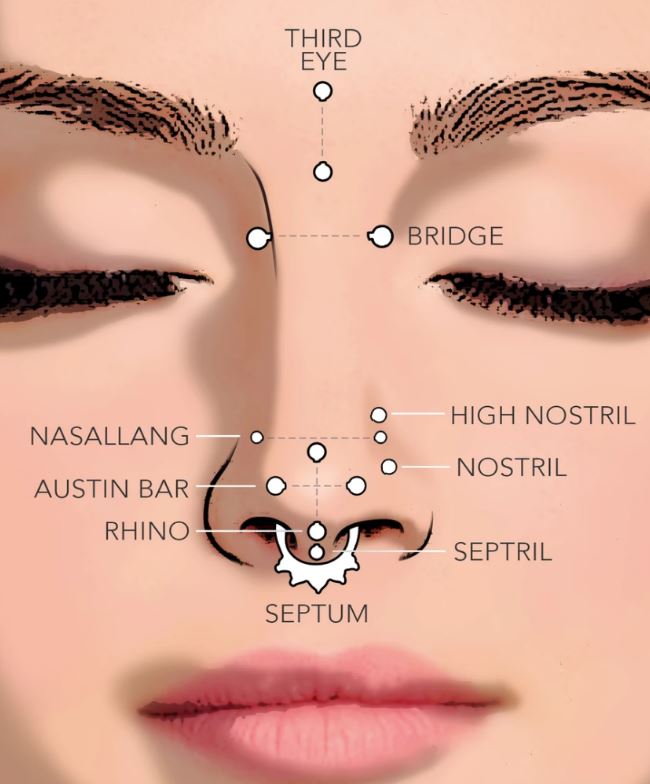
Understanding Nose Piercing Types: A Guide for Teens
Mar 16, 24 09:19 AM
Explore the rising trend of nose piercings among teenagers, understanding the various types and their cultural implications for a stylish appeal. -
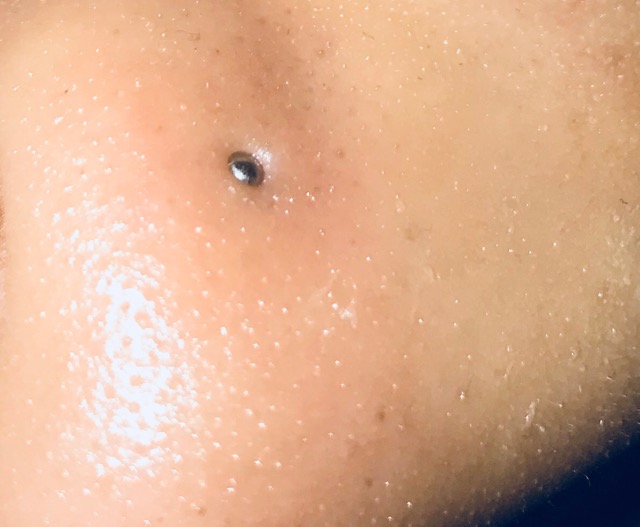
Infected Nose Piercing
Mar 16, 24 09:18 AM
You can expect symptoms of infected nose piercing to resemble any other kind of body piercing infection. -
EMS manufacturing services in Malaysia
Mar 09, 24 10:33 PM
Malaysia is one of the leading countries in Southeast Asia that offers EMS manufacturing services to both local and international clients. -
Laundry Business: The Need for Payment System Upgrades
Mar 08, 24 11:14 AM
Discover the benefits of upgrading your laundry business's payment system. Enhance efficiency, increase profits, and improve customer convenience. -
Nose Peircing Store
Feb 18, 24 02:38 AM
A collection of latest at our nose peircing store. -
How to Choose the Right Coffee Maker for Your Needs
Feb 18, 24 02:12 AM
We'll compare the pros and cons of four common types of coffee makers: drip, French press, espresso, and vacuum. We'll also give you some tips on how to choose the right one based on your preferences… -
Emulate Celebrities with Nose Piercings
Feb 06, 24 08:13 AM
Discover the celebrities with nose piercing and get inspired for your next piercing! From studs to septum rings, our list has it all. Read more! -
Types of Nose Rings
Feb 06, 24 08:11 AM
Types of Nose Rings -
Is my nose piercing ring is sinking in?
Feb 06, 24 08:10 AM
Is my nose piercing ring is sinking in? Or just swollen? -
Dry Herb Vape Pens-Discover the Advantages of Malaysian Made
Feb 04, 24 12:39 PM
Choose our non-China dry herb vape pen for its high production standards, strict quality control, and excellent craftsmanship. -
Trinity Nose Ring A Unique Fashion Statement
Feb 03, 24 08:36 PM
Explore the world of trinity nose rings, a unique piece of jewelry that adds elegance and style to your look. Understand the different types and choose the right one for you. -
Redefining Beauty: The Rise of Nose Piercing Trend in the USA
Feb 02, 24 08:34 AM
Explore the evolution of the nose piercing trend in the USA, from ancient tradition to modern expression of individuality.