Sponsor Ads
Non-China Vape, 510 Cartridges & Battery Device Maker
If you are going for a vape manufacturer out of China we are you best choice. We offer a alternative vape production location with a very competitive price. You can save money by buying directly from us the manufacturer, without any middlemen or extra fees. You can also enjoy discounts for bulk orders and special offers for long term & loyal customers.
We offer small trial orders where you can test the quality and performance of the products before placing a large order. Fast shipping and cheaper shipping cost from Malaysia and Singapore ports. You don't have to wait long to receive your products.
Contact Us!
Contribute for our website Maintenance! We want to keep it free for all visitors.
Trending Best Sellers
Coronavirus Vaccine Update
This Coronavirus Vaccine Update page is intended solely to inform our clients and online visitors about coronavirus COvid-19. All facts are from reliable sources like WHO, CDC and respective Government Health Agencies. Readers can either read through the facts which we summarized in this page or go direct to source through the link listed at the bottom of this page.
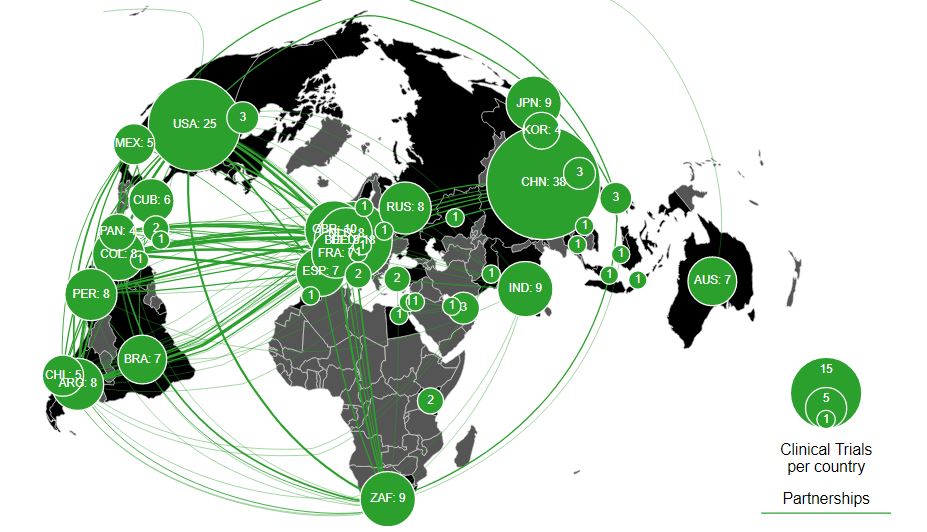
Coronavirus vaccine map (Source: WHO click here for interactive map)
Trending Best SellersGet your latest coronavirus vaccine update! With more than 100 vaccines currently in various trial phases and some reaching the pre-approval stage or being authorized for emergency use, accurate science reporting has never been more important. Journalists play a vital role in informing the public on science, specifically vaccine, developments, in an unprecedented period of scientific publishing.
Latest News on Coronavirus Vaccine
Queen Elizabeth II and husband Prince Philip get COVID-19 vaccine
09/01/2021
Pfizer COVID-19 vaccine appears to protect against UK mutation
The Pfizer/BioNTech COVID-19 vaccine appears to protect against a mutation that has been found in two highly contagious variants of coronavirus which were discovered in the UK and South Africa.
08/01/2021
UK is first country in the world to rollout Oxford-AstraZeneca vaccine last updated: 04/01/2021
British prime minister Boris Johnson met on Monday with some of the first people to receive the new COVID-19 vaccine developed by the University of Oxford and AstraZeneca.Johnson was visiting a London hospital where he observed some healthcare workers receiving their first dose.The UK became the first nation in the world on Monday to start using the new Oxford-AstraZeneca vaccine, ramping up a nationwide inoculation programme as rising infection rates are putting an unprecedented strain on British hospitals.
How a vaccine is approved for production
For more information on the three phases of vaccine clinical trials, click here.
Once a vaccine has reached pre-approval stage following clinical trials, it is assessed by the relevant regulatory body for compliance with quality, safety and efficacy criteria. Following regulatory approval, manufacturers can submit a vaccine to WHO for prequalification (PQ), an assessment process that ensures quality, safety and efficacy and helps the UN and other international procurement organizations determine the programmatic suitability of a vaccine.
During global health emergencies, the WHO Emergency Use Listing Procedure (EUL) may be used to allow emergency use of the vaccine. The EUL exists because, in a pandemic situation, products that could benefit the lives of people all over the world may be prevented from coming to market with sufficient speed. The EUL is a fast-tracked but rigorous process, designed to bring impactful products to all those in need, as quickly as possible, on a time-limited basis and based on a risk-versus-benefit evaluation. The WHO PQ/EUL recommendation may be used by UN agencies such as UNICEF and the Pan American Health Organization Revolving Fund for procurement decisions in low- and middle-income countries. Gavi also relies on WHO EUL/PQ to specify which vaccines its funds may be used to purchase.
WHO issues its first emergency use validation for a COVID-19 vaccine.
31 Dec 2020 (coronavirus vaccine update from WHO)
The World Health Organization (WHO) today listed the Comirnaty COVID-19 mRNA vaccine for emergency use, making the Pfizer/BioNTech vaccine the first to receive emergency validation from WHO since the outbreak began a year ago.
The WHO’s Emergency Use Listing (EUL) opens the door for countries to expedite their own regulatory approval processes to import and administer the vaccine. It also enables UNICEF and the Pan-American Health Organization to procure the vaccine for distribution to countries in need.
Regulatory experts convened by WHO from around the world and WHO’s own teams reviewed the data on the Pfizer/BioNTech vaccine’s safety, efficacy and quality as part of a risk-versus-benefit analysis. The review found that the vaccine met the must-have criteria for safety and efficacy set out by WHO, and that the benefits of using the vaccine to address COVID-19 offset potential risks.
The vaccine is also under policy review. WHO’s Strategic Advisory Group of Experts on Immunization (SAGE) will convene on 5 January, 2021, to formulate vaccine specific policies and recommendations for this product’s use in populations, drawing from the SAGE population prioritization recommendations for COVID-19 vaccines in general, issued in September 2020.
Further resources: for Coronavirus Vaccine Update
WHO’s new COVID-19 vaccines page includes WHO news and resources, explainers on key topics, and answers to frequently asked questions.
Pan American Health Organization (PAHO): COVID-19, An Informative Guide: Advice for Journalists
WHO situation reports: The Weekly Epidemiological Update provides an overview of the global, regional and country-level COVID-19 cases and deaths, highlighting key data and trends, as well as other pertinent epidemiological information concerning the COVID-19 pandemic.
Journalism in a Pandemic: Covering COVID-19 Now and in the Future is a free, self-directed online course produced by the Knight Center for Journalism in the Americas in collaboration with WHO, UNESCO, and UNDP; it includes a module on COVID-19 treatment and vaccine issues.
A database of expert sources on COVID-19 for journalists to contact for up-to-date information on the outbreak. This includes governmental departments and agencies for some of the worst-affected countries, as well as regional and global health organizations.
Important Covid-19 Links
This Coronavirus pages are intended solely to inform our clients and online visitors about coronavirus/COvid-19. All facts are from reliable sources like WHO, CDC and respective Government Health Agencies. Readers can either read through the facts which we summarized in this page or go direct to source through the link listed at the bottom of each page.
- Safety of COVID-19 vaccines-what we need to know!
- Coronavirus - Stay Informed
- Covid 19
- Covid 19 Symptoms
- Coronavirus Covid 19 Questions and Answers
- Coronavirus disease (COVID-19) advice for the public
- Coronavirus Vaccine - All you need to know!
- Covid 19 Testing Near Me
- Coronavirus Vaccine
- What is the Coronavirus Vaccine
- Coronavirus vaccine update - There are more than 100 vaccines!
- How long does coronavirus last - Stay Informed!
- All About Covid-19
- Covid-19 Symptoms
- Michigan Coronavirus _ Stay Informed and Stay Safe!
- Coronavirus California - Stay Informed & Stay Safe
- Florida coronavirus - Stay Informed!
- What is the coronavirus vaccine - Stay Informed
- Covid 19 Vaccine - Stay Informed with the latest Development!
- Protect yourself: advice for the public
- Myth busters
- Questions and answers
- Situation reports
- All information on the COVID-19 outbreak
References:
1. WHO Coronavirus disease (COVID-19) pandemic
2. CDC Centers for Disease and Prevention Control
3. Canada Coronavirus disease (COVID-19)
4. Health.com
5. Harvard Health Publishing Harvard Medical School
7. Wikipedia COVID-19 pandemic
Supply Chain
1) Nitrile Powder-Free Examination Gloves
3) Blue Nitrile Disposable Gloves Powder-Free
Coronavirus Vaccine Update Disclaimer
Disclaimer: the designations employed and the presentation of the material in publications listed in this database does not imply the expression of any opinion whatsoever on the part of NosePiercings.Com concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted and dashed lines on maps represent approximate border lines for which there may not yet be full agreement.
The mention of specific companies or of certain manufacturers’ products in publications listed in the database does not imply that they are endorsed or recommended by NosePiercings.Com in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters.
By listing publications in this database and providing links to external sites does not mean that NosePiercings.Com endorses or recommends those publications or sites, or has verified the content contained within them. The database has been compiled without warranty of any kind, either expressed or implied. The responsibility for the interpretation and use of publications included in this database lies with the reader. In no event shall NosePiercings.Com be liable for damages arising from its use.
To the top of this Coronavirus Vaccine Update page.
Comments
What you think?
Recent Articles
-
Riche Niche: Health | Lifestyle | Fashion | Marketing | Technology
Mar 14, 25 09:18 AM
Our Riche Niche blog is the easiest way to stay up-to-date with the latest news, trends and articles published on this site. -
The Therapeutic Potential of Medical Cannabis Vaporization
Aug 05, 24 09:32 PM
The use of medical cannabis has been a subject of much debate and research over the years. With the growing acceptance of cannabis for medical purposes, various methods of administration have been exp… -
Amazon Spring Sale: A Season of Spectacular Savings
Mar 18, 24 08:38 AM
Amazon Spring Sale: A Season of Spectacular Savings -
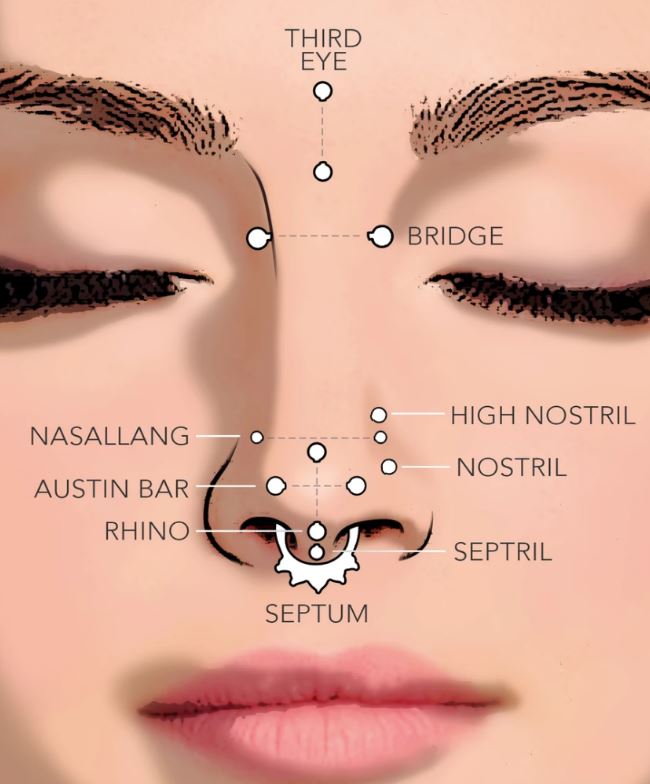
Understanding Nose Piercing Types: A Guide for Teens
Mar 16, 24 09:19 AM
Explore the rising trend of nose piercings among teenagers, understanding the various types and their cultural implications for a stylish appeal. -
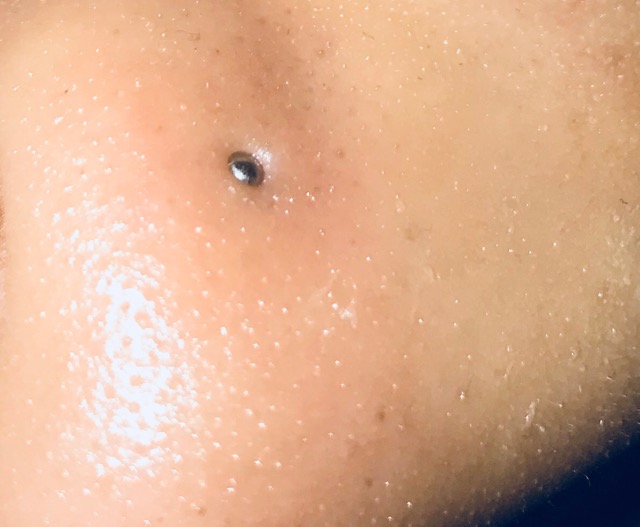
Infected Nose Piercing
Mar 16, 24 09:18 AM
You can expect symptoms of infected nose piercing to resemble any other kind of body piercing infection. -
EMS manufacturing services in Malaysia
Mar 09, 24 10:33 PM
Malaysia is one of the leading countries in Southeast Asia that offers EMS manufacturing services to both local and international clients. -
Laundry Business: The Need for Payment System Upgrades
Mar 08, 24 11:14 AM
Discover the benefits of upgrading your laundry business's payment system. Enhance efficiency, increase profits, and improve customer convenience. -
Nose Peircing Store
Feb 18, 24 02:38 AM
A collection of latest at our nose peircing store. -
How to Choose the Right Coffee Maker for Your Needs
Feb 18, 24 02:12 AM
We'll compare the pros and cons of four common types of coffee makers: drip, French press, espresso, and vacuum. We'll also give you some tips on how to choose the right one based on your preferences… -
Emulate Celebrities with Nose Piercings
Feb 06, 24 08:13 AM
Discover the celebrities with nose piercing and get inspired for your next piercing! From studs to septum rings, our list has it all. Read more! -
Types of Nose Rings
Feb 06, 24 08:11 AM
Types of Nose Rings -
Is my nose piercing ring is sinking in?
Feb 06, 24 08:10 AM
Is my nose piercing ring is sinking in? Or just swollen? -
Dry Herb Vape Pens-Discover the Advantages of Malaysian Made
Feb 04, 24 12:39 PM
Choose our non-China dry herb vape pen for its high production standards, strict quality control, and excellent craftsmanship. -
Trinity Nose Ring A Unique Fashion Statement
Feb 03, 24 08:36 PM
Explore the world of trinity nose rings, a unique piece of jewelry that adds elegance and style to your look. Understand the different types and choose the right one for you. -
Redefining Beauty: The Rise of Nose Piercing Trend in the USA
Feb 02, 24 08:34 AM
Explore the evolution of the nose piercing trend in the USA, from ancient tradition to modern expression of individuality.